Which Of The Following Best Describes The Noble Gases
Which of the following best describes the noble gases. Which of the following best describes the transition elements. The solid warms until it. The outer shell of valence electrons is considered to be full in noble gases giving them little tendency to participate in chemical reactions.
Combine easily with other elements. As the atomic number of the elements in the graph increases each elements atomic radius also increases. A H 2 Br 2 2HBr B H 2 Br 2 HBr C H 2 2Br 2 2HBr D 2H 2 Br 2 HBr 1 Which of the following pieces of glassware can be used to measure the volume of a.
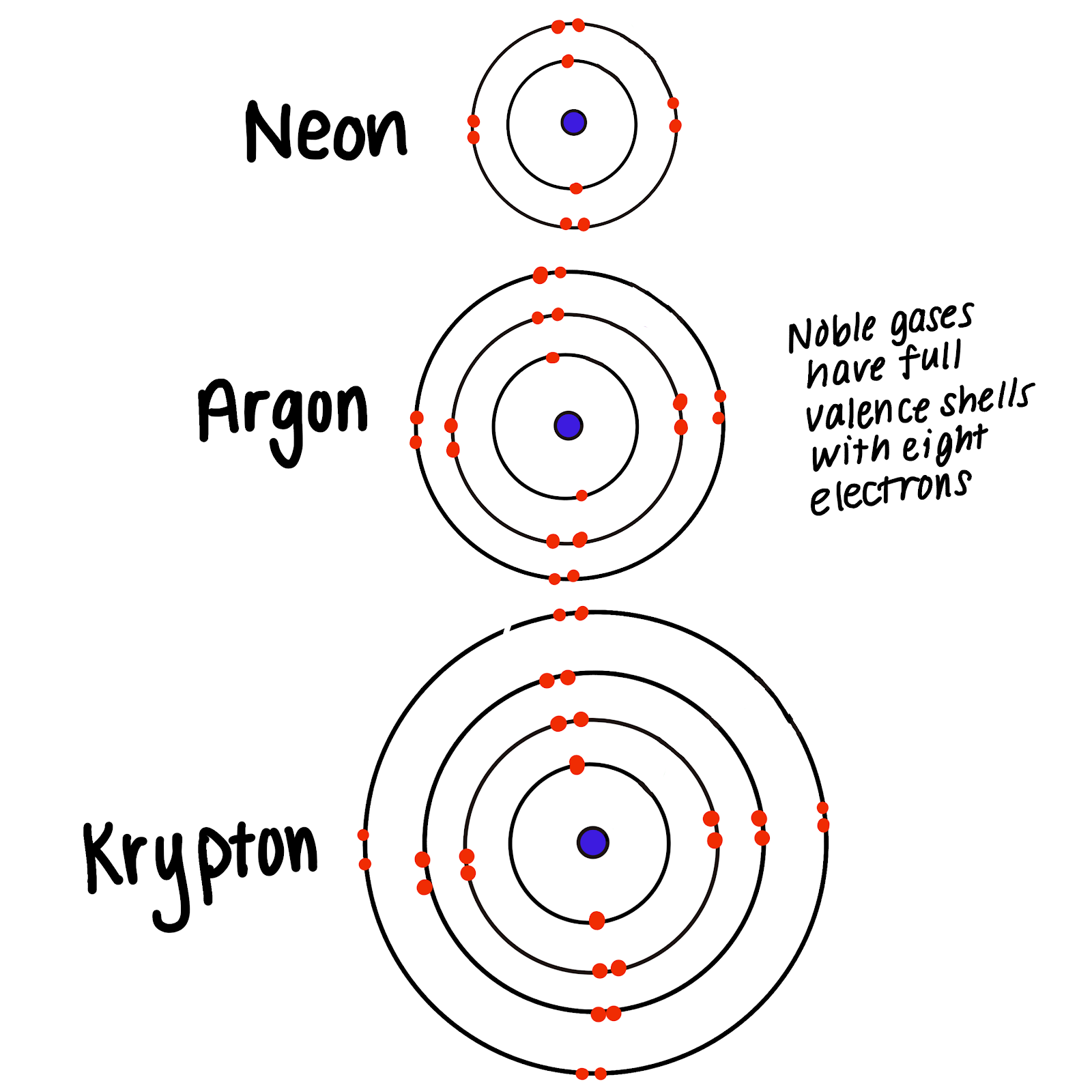
Only the noble gases the elements on the right-most column of the periodic table have zero charge with filled valence octets. The electron valence shell of all these elements is full. Consider this model for one of the noble gases to answer the following questions.
Other characteristics of the noble gases are that they all conduct electricity fluoresce are odorless and colorless and are used in many conditions when a. The innermost shell only holds two electrons so Helium is usually also called a noble gas because it is not reactive for the same reason of a full outer electron shell. Neon Argon etc have full outer shells with eight.
Then mark the space on the answer sheet for the answer you have chosen. This means they have an octet configuration. Compare the outermost electron in each of the following atoms.
He has two electrons wich is the maximum an atom of the first row can have. Moving down the group in the periodic. Which of the following best describes the phase changes that occur when the temperature of CO2 is increased from -100C to 25C at a pressure of 1atm.
He has two electrons wich is. Noble gases is the family name given to the elements of group 18 of the periodic table.
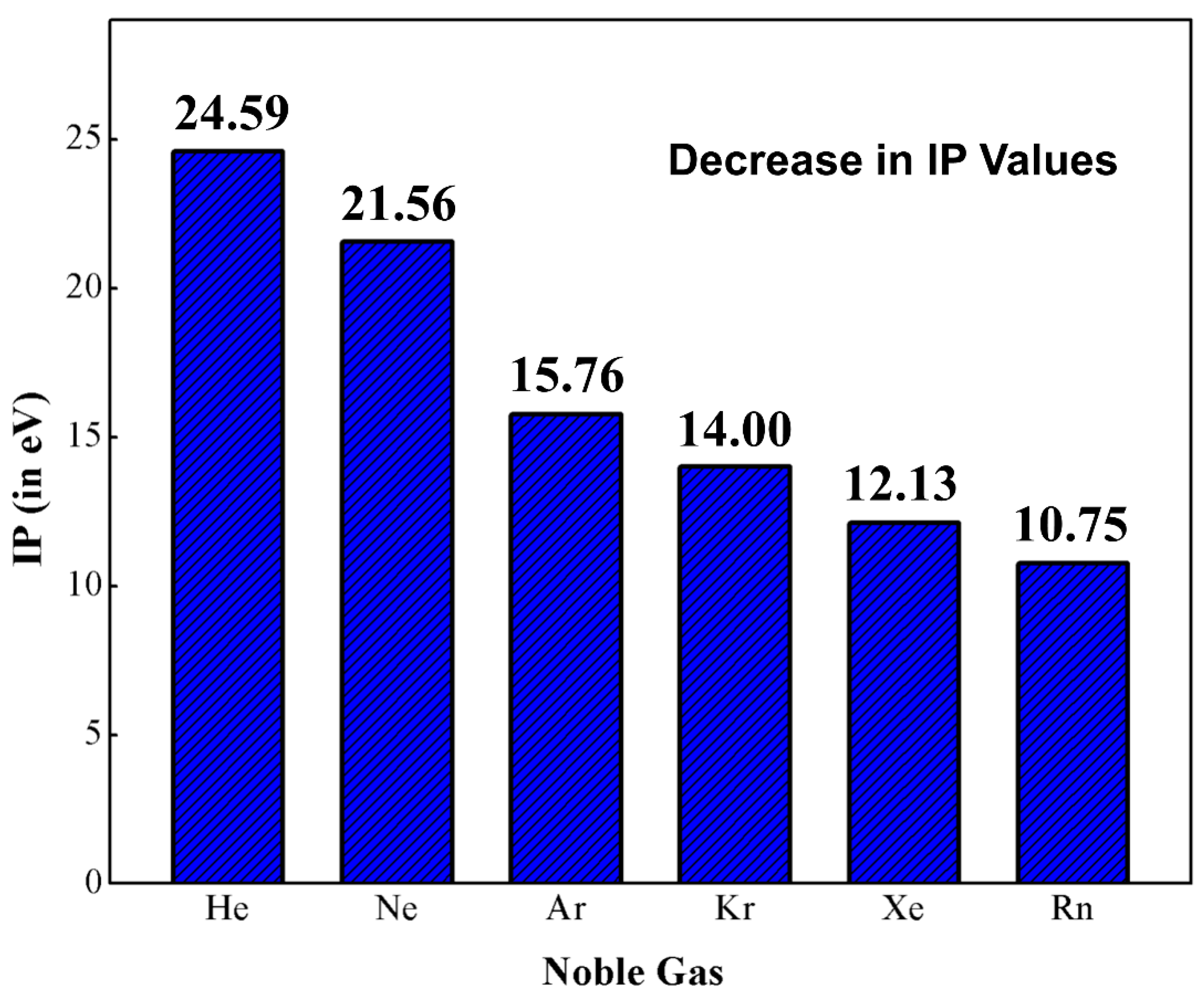
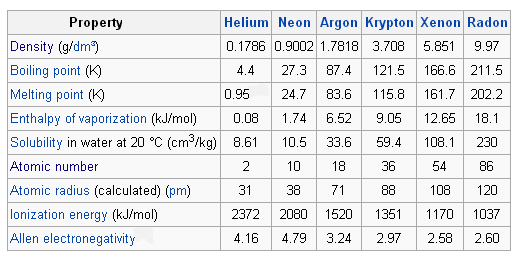
Each element is non-reactive has high ionization energy electronegativity near zero and a low boiling point.
Neon Argon etc have full outer shells with eight. Compare the outermost electron in each of the following atoms. Helium neon and argon. The noble gases have also been referred to as inert gases but this label is deprecated as many noble gas compounds are now known. Neon Argon etc have full outer shells with eight. Which list of elements belongs to the family commonly called the Noble Gases. Rare gases is another term that was used 4 but this is also inaccurate because argon forms a fairly considerable part 094 by volume 13 by mass of the Earths atmosphere due to decay of radioactive. All of the other elements have a charge when they have eight electrons all to themselves. The graph below shows atomic radii of the noble gases.
The solid warms until it. The innermost shell only holds two electrons so Helium is usually also called a noble gas because it is not reactive for the same reason of a full outer electron shell. Noble gases is the family name given to the elements of group 18 of the periodic table. Which of the following best describes the noble gases. Other characteristics of the noble gases are that they all conduct electricity fluoresce are odorless and colorless and are used in many conditions when a. He has two electrons wich is. A 1300 mL B 1350 mL C 1400 mL D 1450 mL 15 10 5 VASpr08 EOC Chem RB 32808 839 AM Page 4.































/krypton-laser-beams-520689060-579fdd6f5f9b589aa9edb484.jpg)
/metals-versusnonmetals-608809-v3-5b56348946e0fb0037001987.png)



Posting Komentar untuk "Which Of The Following Best Describes The Noble Gases"